Two American and German companies announced that they will soon have a Covid-19 vaccine 5
`It has an excellent track record and I consider this vaccine to be almost perfect,` Ugur Sahin, CEO and co-founder of German biotechnology company BioNTech said today about BNT162, the Covid-19 vaccine.
The two companies said they plan to supply 100 million doses of BNT162 by the end of this year and about 1.3 billion doses next year.
In July, the US Department of Health and Human Services and the Department of Defense announced a $1.95 billion agreement with Pfizer to produce 100 million doses of Covid-19 vaccine.
Sahin shared that he believes that emergency use authorization for this vaccine candidate will be quickly approved by authorities, adding that `understanding of how it works along with safety data from the process
`We believe we will have a safe product and we also believe we can prove it is effective,` he said.
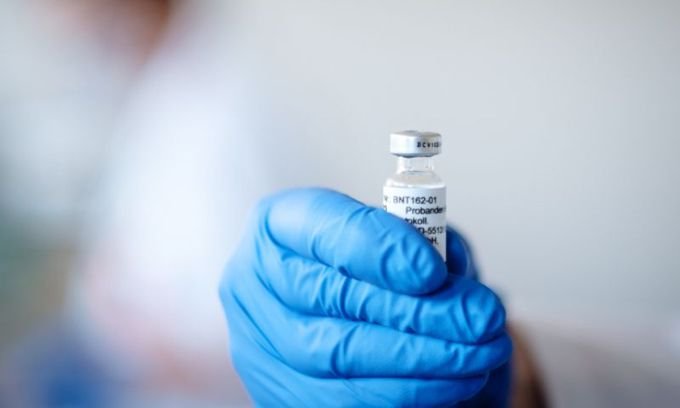
A prototype of the BNT162 vaccine developed by Pfizer and BioNTech.
BioNTech’s CEO said clinical trials in elderly and young adults showed the antibody response was robust, with minimal side effects.
`Only a small portion of the trial participants had a fever,` Sahin said.
Although BioNTech and Pfizer believe their vaccine will be approved by mid-October, many federal officials believe this is an `overly optimistic` timeline.
`I have not seen any scientist involved in the vaccine development effort think that we will have shots before the November 3 election,` said an official familiar with the government’s rapid vaccine development project.
Song Moncef Slaoui, chief advisor to Operation Warp Speed, told NPR last week that it is `extremely unlikely but not impossible` for a Covid-19 vaccine to be approved by the US Food and Drug Administration (FDA).
US President Donald Trump has also repeatedly said that he is `optimistic` about a vaccine being approved for use before November 3.
BioNTech and Pfizer are two of nine pharmaceutical companies that have signed a pledge to maintain `high ethical standards`, which states they cannot ask the government for early approval of vaccines.
However, Sahin said that the two companies will quickly apply for a license for candidate BNT162 because they believe the full process has been completed.
`I believe that with us having completed the full process required for vaccine development including scientific research, pre-clinical research, safety acceptance testing and efficacy testing, there is no need to
BioNTech and Pfizer’s BNT162 is one of 34 vaccines undergoing clinical trials globally, according to the World Health Organization.







